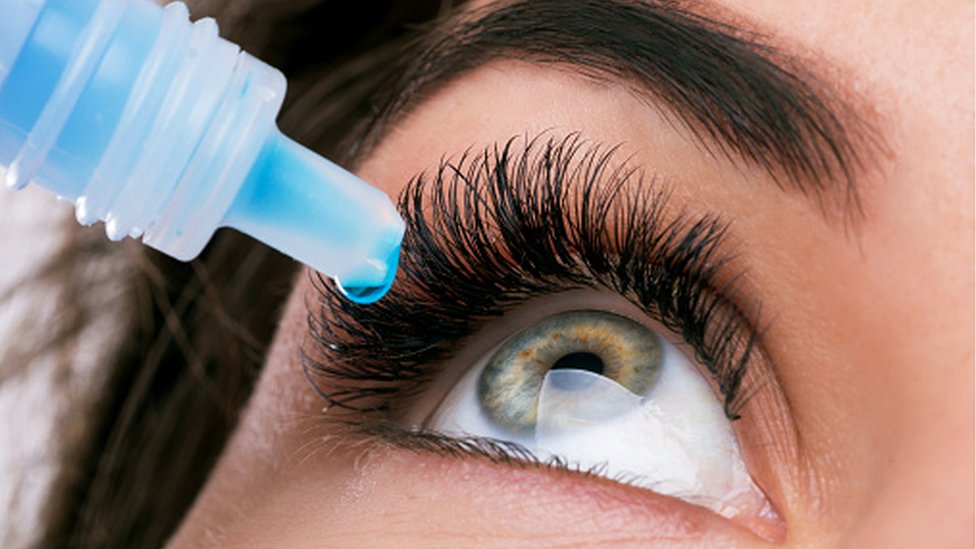
Consumers using eye drops from South Moon, FivFivGo, or Rebright are advised not to use or purchase these goods as they are imitations and may cause eye infections, according to the United States Food and Drug Administration (FDA). Bausch + Lomb’s Lumify brand eye drops, which are available over-the-counter and have FDA approval for treating redness, are readily mistaken with the fake eye drops. The unapproved eye drop products shouldn’t be sold in the United States despite their promises to treat glaucoma and other illnesses that call for medication or surgery.
Risks Associated with Copycat Products
Vicente Diaz, M.D., M.B.A., Chief of Ophthalmology at Yale School of Medicine, has cautioned against the potential risks associated with counterfeit eye products, emphasizing the dangers despite the temptation to purchase them. He pointed out that these imitations are manufactured by smaller enterprises, which lack the level of regulatory oversight in their manufacturing practices. Dr. Diaz highlighted a recent incident involving artificial tear manufacturers facing similar issues, leading to severe consequences such as blindness and fatalities.
FDA Identifies Counterfeit Products
In its warning, the FDA named three distinct counterfeit goods—South Moon, Rebright, and FivFivGo—that were impersonating Bausch + Lomb’s Lumify drops. The imitations’ packaging, which has an illustration of an eye with a light purple iris and white text on a gray backdrop, is designed to closely mimic that of the original Lumify drops. The bottles within the package are identical to those of Lumify, with a gray label with white text and a purple cap.
Dangers of Silver Compounds
“Some of these illegally marketed, unapproved products have silver compounds which can cause discoloration of the eye, skin and other tissues, causing them to turn gray or blue-gray,” said Dr. Brian Boxer Wachler, ophthalmologist and medical reviewer at All About Vision. According to the FDA, those who use these illegal eye products run the risk of postponing or discontinuing safe and efficient medical procedures.
Within 15 days of receiving the warning letter, the FDA has urged the firms to provide a response outlining their plans to address the infractions. According to the government, if the firm doesn’t make the necessary changes, there might be legal repercussions, such as product seizure or a court order compelling the company to cease producing and selling the prohibited eye solution.

Guidance for Safe Purchases
The FDA advises customers to buy eye products from respectable merchants, such as pharmacies with state licenses. Also, use caution when purchasing items from internet merchants who provide false information. Diaz suggests the following brands of eye drops when searching for trustworthy ones: Refresh and Systane are the top options for dry eyes. Bausch & Lomb’s Lumify, a brimonidine-based medicine with no known production concerns, is a well-known brand for treating eye redness.
FDA Issues Warning Letters
The Food and Drug Administration sent warning letters to CVS, Walgreens, and a number of other businesses for producing or distributing potentially hazardous, unapproved eye products. The eye ailments that the items claim to address include glaucoma, cataracts, and conjunctivitis, sometimes known as “pink eye.” A few of the letters discuss problems with product sterility and quality. If a customer is utilizing any of these products, they should consult their physician. The FDA’s MedWatch programme is where anyone may report suspected side effects from using a product.

Following a drug-resistant epidemic that afflicted dozens of patients last year, four of whom died from illnesses, the FDA has increased warnings about eye products. The outbreak was associated with different eye drops, even though EzriCare Artificial Tears were the most often reported exposure.



