Dust scattered across the moon may hold clues to the mysteries of supernova star explosions, and researchers at the China Institute of Atomic Energy (CIAE) have developed a novel method for deciphering these signs of stellar death.
The discovery may offer scientists a better understanding of how stars die and provide material for subsequent generations of stars, planets, moons, and occasionally even life.
The method is based on the enhanced identification of a rare iron isotope that is minutely present in lunar dust. Millions of years ago, the hearts of earlier generations of huge stars created this type of iron. These stars’ millions or billions of years-long “tug of war” battles with gravity ended in supernova explosions, which released the isotopes, scattering them throughout space and possibly even landing on the moon, according to scientists.
Team leader and CIAE researcher Bing Guo said, “Our team came to the conclusion that pushing the limits of what our equipment could do was the only way to track earlier supernova explosions accurately.”
Supernovas contribution to cosmic recycling
(Image credit: STsci)
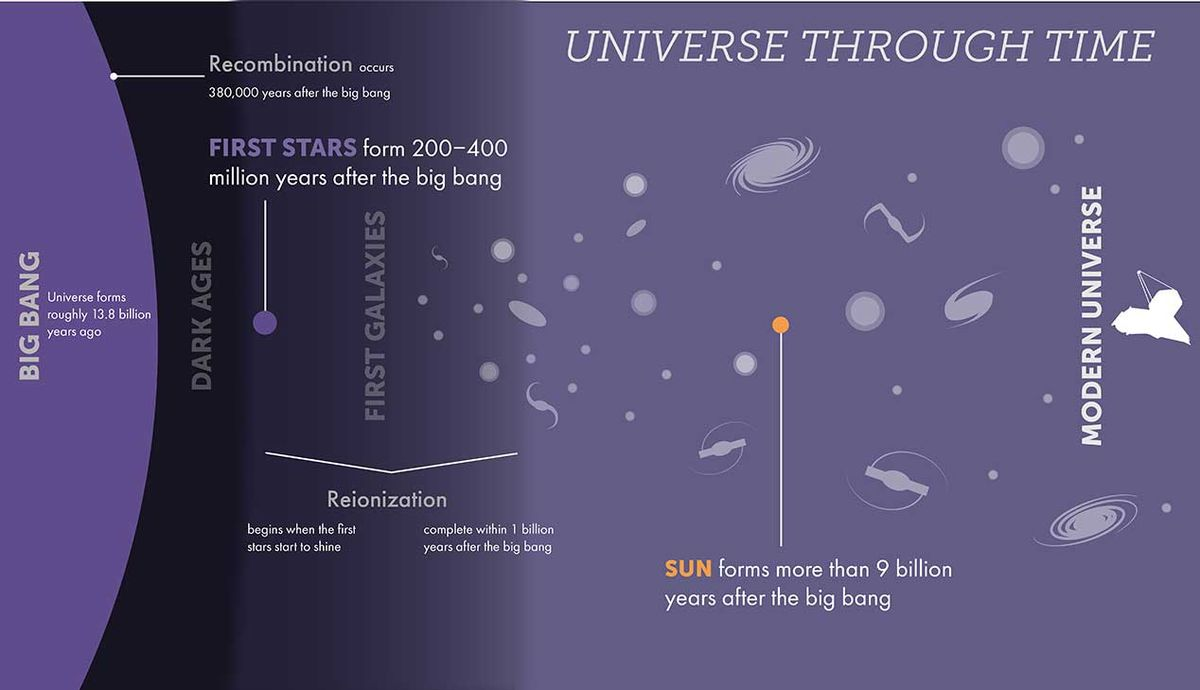
Between 200 million and 400 million years after the Big Bang, the cosmos was primarily composed of hydrogen, with a small amount of helium, when the first generation of stars formed. This is the lightest element that exists now, and astronomers (confusingly) refer to these as “metals.”
This indicates that hydrogen, helium, and very few metals made up the early stars, which are surprisingly referred to as Population III stars. These stars’ ability to convert hydrogen into helium through nuclear fusion processes in their cores gave them the ability to shine brightly in space while they were alive. The outward radiation pressure produced by this fusion process also kept them from collapsing due to the inward force of their own gravity.
However, this meant that the balance between radiation pressure and gravity ended with gravity clearly winning when hydrogen ran out in the core of these stars. As a result, the outer layers of these stars, where nuclear fusion was still occurring, were blown away while the cores imploded.
When a star’s mass approaches that of the sun, its stellar cores cool and eventually disperse, transforming into white dwarf stars with a cloud of once-stellar material surrounding them. However, stars that have at least eight times the mass of the sun will not suffer that destiny.
The pressure created in these huge stars’ cores during their collapse causes helium to nuclear fusion into other heavier elements. This process is repeated in the most massive stars until iron, the heaviest material that a star can conceivably manufacture, fills the core.
Following this, the core of a large star will collapse once more, resulting in the explosion of a supernova. All of the elements that the star has produced over its lifetime are released by that explosion and spread over the nearby galaxy. After then, the star transforms into a dense stellar remnant, which can either be a black hole in situations of total gravitational collapse or a neutron star.
But the elements the star generated during its lifespan don’t end there. These substances end up in gas and dust interstellar clouds, which have the potential to collapse and give rise to stars and planets.
That is how later stellar generations became progressively more “metal-rich” over time. All of the scattered material is also incorporated into the developing planets orbiting these stars and any potential life forms on those worlds. Therefore, when scientists say, “You are star stuff,” they are not just saying it; they are saying it with fact.
The moon-dust-tracking team is looking for a rare isotope produced following a supernova, which is a better tracer of this cosmic recycling process than an element formed during the star’s lifespan.
Supernovas to the Moon: Iron-60
Supernovas unleash as much energy during their explosion in a matter of seconds as the sun would take billions of years to radiate. This creates the ideal environment for creating heavy radioactive isotopes. The group’s goal is to enhance methods for searching lunar dust for “iron-60,” a radioactive form of iron.
Iron-60 has a half-life of about 2.3 million years and an atomic nucleus with 26 protons and 34 neutrons. Iron-60 can be produced in supernovae in quantities up to ten times the mass of Earth, although the solar system produces very little of this isotope. According to scientific predictions, “nearby” star explosions coincide with supernovae approximately three times every 100 years throughout the Milky Way.
According to the research team, finding iron-60 on Earth or the moon is a good indicator of a supernova erupting in the recent past of our 4.6 billion-year-old planet, reasonably close to the solar system, say, within about 100 light-years.
But iron-60 is rare, and the presence of other, more ubiquitous interfering elements makes it very difficult for low-sensitivity spectrometers to detect its low abundance. Guo and associates modified the CIAE’s HI-13 tandem accelerator facility in order to counteract this. In order to perform “accelerator mass spectrometry” (AMS), a “Wein filter,” a tool that may be used to select charged particles moving at particular speeds, had to be added.
The researchers discovered that AMS could identify iron-60 in synthetic samples with a sensitivity that was far higher than that of the equipment usually employed in similar investigations.
The CIAE team thinks they can now further extend the AMS system’s detection sensitivities, which could significantly advance our knowledge of stars that perished in supernova explosions and prolong human life.
“The deployment of the Wien filter could be an important turning point for us,” Guo stated. “Our next objective is to further reduce detection limits by optimising our complete AMS system. A world of opportunities becomes available with each degree of heightened sensitivity.”
The study’s findings were released in the journal Nuclear Science and Techniques on May 24.



