On Sunday, Bharat Biotech announced that it has started the Tuberculosis (TB) vaccine MTBVAC trials in India.
This is the Phase-3 trial for the first human source-derived TB vaccine, developed by a Spanish Biopharmaceutical company, Biofabri, in collaboration with Bharat Biotech International Ltd.
The trials aim to evaluate the safety and effectiveness of MTBVAC.
Table of Contents
MTBVAC TB Vaccine
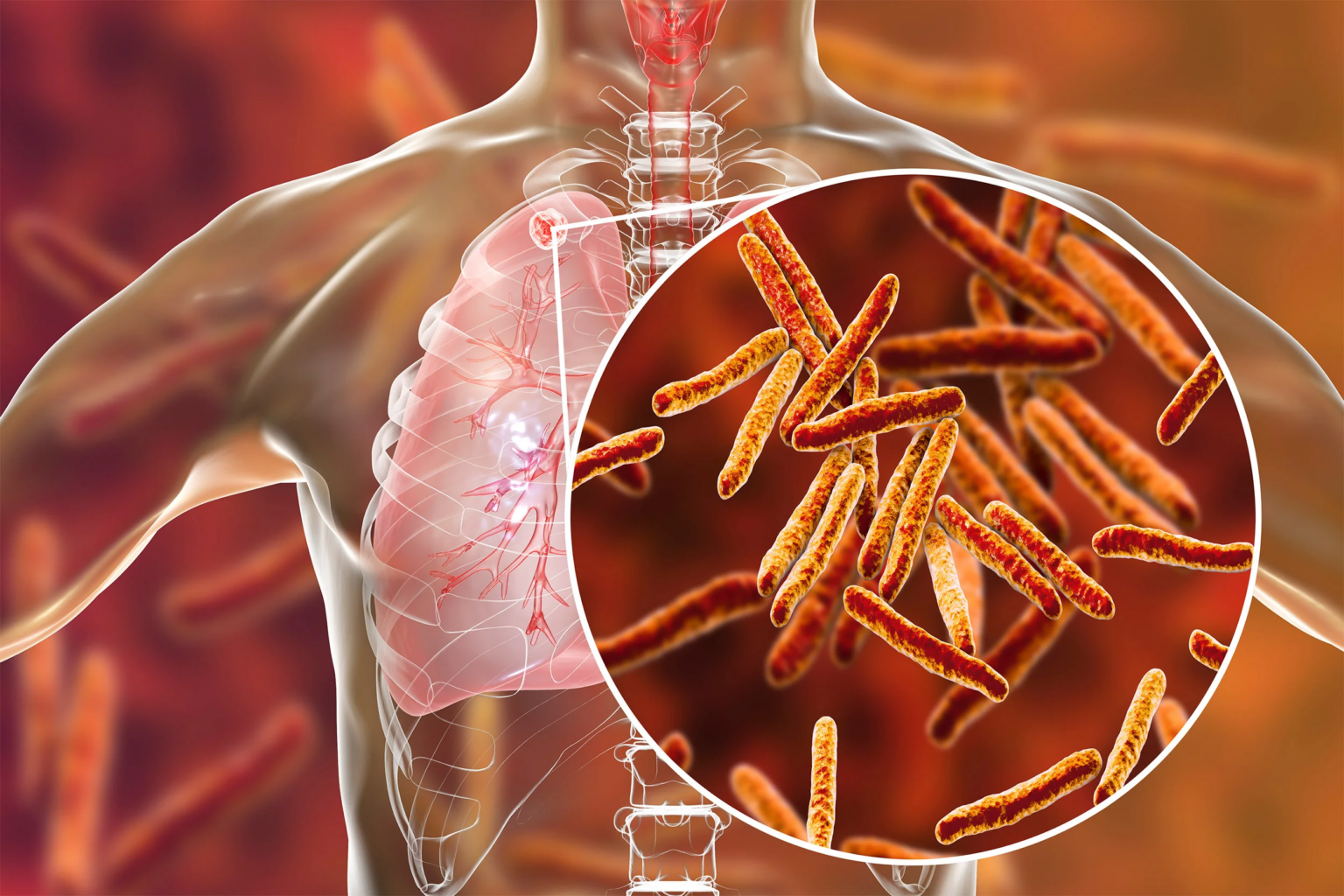
Tuberculosis, also known as TB, is an infectious air-borne disease caused by bacteria that mainly affects the lungs. It is a preventable disease and can be treated.
According to WHO, around 25% of the world’s population has contracted TB bacteria. However, only in 5-10% of the infected people the bacteria develop TB. In 2022, TB caused 1.3 million deaths worldwide, which makes it the second-leading cause of death by infectious disease after COVID-19.
Certain antibiotics can kill Tb bacteria faster than they multiply. However, the bacteria can develop multi-drug resistance through a protective coating that can block many drugs, making it difficult for the immune system to fight them. Many multi-drug resistance strains have emerged recently, highlighting the need for a potent TB vaccine.
Traditionally, the only vaccine used to prevent TB is Bacille Calmette-Guerin (BCG), obtained from a weak strain of Mycobacterium bovis. It is primarily given to newborns.
Although the BCG vaccine helps in reducing the spread of TB in children, it is not effective in preventing pulmonary TB. It is the most common disease in teenagers and adults and is responsible for spreading it to others.
MTBVAC is a new TB vaccine that is being tested in adults. It is made from a weakened strain of Mycobacterium tuberculosis, a bacteria that causes TB in humans.
The MTBVAC TB vaccine was created by Dr. Brigitte Gicquel from the Pasteur Institute, Paris, in the laboratory of the University of Zaragoza.
Bharat Biotech, in partnership with Biofabri, responsible for the vaccine’s industrial development, has begun Phase-3 trials in India. However, a safety, immunogenicity, and efficacy trial will start in 2025.
Previously, the Phase 1 and 2 trials were conducted in South Africa, Madagascar, and Senegal.
India’s Role in the MTBVAC TB Vaccine’s Progress
According to the WHO, over 80% of the cases and deaths due to TB are in low and middle-income countries.
In 2022, around 87% of the new TB cases were recorded in 30 countries with the highest disease burden. More than two-thirds of the cases were from Bangladesh, China, the Democratic Republic of Congo, India, Indonesia, Nigeria, Pakistan, and the Philippines.
India reported over 24.22 lakh TB cases in 2022, surpassing the pre-covid numbers with the help of governmental efforts.
On such grounds, it is essential to study the safety, effectiveness, and ability of the MTBVAC TB vaccine to produce an immune response in India, especially as a country with the largest population and a high number of cases of TB.
Biofabri CEO Esteban Rodriguez says, “It is a giant step to test in adults and adolescents in the country where 28% of the world’s TB cases accumulate,”
MTBVAC TB vaccine aims to be a more potent and longer-lasting vaccine than BCG for newborns and to prevent it in teenagers and adults.
Krishna Ella, Executive Chairman of Bharat Biotech, said in the press release that the quest for a more effective vaccine against tuberculosis, with clinical trials in India, received a big boost today. The MTBVAC vaccine has passed several milestones before entering clinical trials in India
Sources suggest that Bharat Biotech is given exclusive rights for industrial manufacture of the MTBVAC TB vaccine for newborns, teenagers, and adults.
The development of MTBVAC is a collaborative effort between public and private organizations. International AIDS Vaccine Initiative (IAVI) and the National Institutes of Health have contributed to its funding and conducting the phases of trials across countries.